Answer : The formic acid pressure is, 99 torr
Explanation :
The given chemical reaction is:
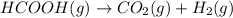
Initial pressure a 0 0
At time 't' (a-x) x x
According to the Dalton's law,
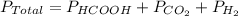
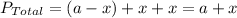 .........(1)
.........(1)
As we are given that:
Initial pressure = a = 220 torr
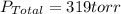
Now put the value of 'a' in equation 1, we get:
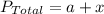
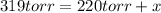
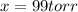
Thus, the formic acid pressure is, 99 torr