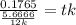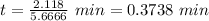Answer:
0.3738 min
Step-by-step explanation:
Integrated rate law for second order kinetics is:
![(1)/([A_t]) = (1)/([A]_0)+kt](https://img.qammunity.org/2021/formulas/chemistry/college/bj32u0ycxy039bxo2npb8w4kujg1ya1aj4.png)
Where,
![[A_t]](https://img.qammunity.org/2021/formulas/chemistry/high-school/c6se0yk0a5jz0ud2m1a9jh5tv0rk9jx59i.png) is the final initial concentration
is the final initial concentration
![[A_0]](https://img.qammunity.org/2021/formulas/chemistry/high-school/i49y9xugeve1tuhjmf05tpufcmfey5f0yu.png) is the initial concentration
is the initial concentration
k is the rate constant
t is the time
Given that:-
85% is complete which means that 0.85 of
![[A_0]](https://img.qammunity.org/2021/formulas/chemistry/high-school/i49y9xugeve1tuhjmf05tpufcmfey5f0yu.png) is decomposed. So,
is decomposed. So,
![{[A_t]}](https://img.qammunity.org/2021/formulas/chemistry/college/qv5g58a9m29de8ozw1qbyohlda0hesep5w.png) = 1 - 0.85
= 1 - 0.85
![[A_0]](https://img.qammunity.org/2021/formulas/chemistry/high-school/i49y9xugeve1tuhjmf05tpufcmfey5f0yu.png) =
=
![0.15[A_0]](https://img.qammunity.org/2021/formulas/chemistry/college/jgudxgcdkwdfdens21g9dyuin43vha2u4p.png)
t = 12 minutes
![(1)/(0.15[A_0]) = (1)/([A]_0)+k* 12](https://img.qammunity.org/2021/formulas/chemistry/college/civt1r3rjwuol1ptz1hlqnbdskwqa0c5gq.png)
![(5.6666)/([A_0]) =12k](https://img.qammunity.org/2021/formulas/chemistry/college/nksmrm3xgpx2ofmm76w4byy7d6f70ufqri.png)
![[A_0]=(5.6666)/(12k)](https://img.qammunity.org/2021/formulas/chemistry/college/w03uncbkyr7dwyns572f7gfp57sah2ho0i.png) - 1
- 1
Also,
15 % is complete which means that 0.15 of
![[A_0]](https://img.qammunity.org/2021/formulas/chemistry/high-school/i49y9xugeve1tuhjmf05tpufcmfey5f0yu.png) is decomposed. So,
is decomposed. So,
![{[A_t]}](https://img.qammunity.org/2021/formulas/chemistry/college/qv5g58a9m29de8ozw1qbyohlda0hesep5w.png) = 1 - 0.15
= 1 - 0.15
![[A_0]](https://img.qammunity.org/2021/formulas/chemistry/high-school/i49y9xugeve1tuhjmf05tpufcmfey5f0yu.png) =
=
![0.85[A_0]](https://img.qammunity.org/2021/formulas/chemistry/college/29ipa47ox1hj0b7regnmgpal7yoopupi5w.png)
t = ?
![(1)/(0.85[A_0]) = (1)/([A]_0)+kt](https://img.qammunity.org/2021/formulas/chemistry/college/pvejscvys1vb1pn1hinl4ujj8nofk6xsgp.png)
![(0.1765)/([A_0]) =tk](https://img.qammunity.org/2021/formulas/chemistry/college/4xi7d8kg4yn7lrq7bsva9hahatw68wiilf.png)
Applying value from 1