Answer : The concentration of solution is, 8.53 M.
Explanation :
As we are given, 45.0 mass % solution of ethanol in water that means 45.0 g of ethanol present in 100 g of solution.
First we have to calculate the volume of solution.
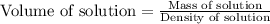
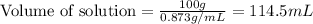
Now we have to calculate the molarity of solution.
Mass of
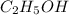 = 45.0 g
= 45.0 g
Volume of solution = 114.5 mL
Molar mass of
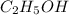 = 46.07 g/mole
= 46.07 g/mole
Molarity : It is defined as the number of moles of solute present in one liter of volume of solution.
Formula used :
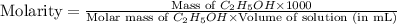
Now put all the given values in this formula, we get:
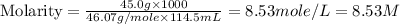
Therefore, the concentration of solution is, 8.53 M.