Answer:
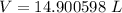
Volume of the balloon at a temperature of 24 C is 14.00598 L
Step-by-step explanation:
The formula we are going to use is:
PV=nRT
Where;
P is the pressure
V is the volume
n is the number of moles
R is the gas constant
T is the temperature
Calculating n:
n=
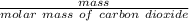
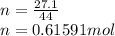
T=24+273
T=297 K
P=
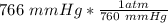
P=1.00789 mmHg
R=0.0821 L atm/mol.K

Volume of the balloon at a temperature of 24 C is 14.00598 L