Here is the complete question.
Benzalkonium Chloride Solution ------------> 250ml
Make solution such that when 10ml is diluted to a total volume of 1 liter a 1:200 is produced.
Sig: Dilute 10ml to a liter and apply to affected area twice daily
How many milliliters of a 17% benzalkonium chloride stock solution would be needed to prepare a liter of a 1:200 solution of benzalkonium chloride?
(A) 1700 mL
(B) 29.4 mL
(C) 17 mL
(D) 294 mL
Answer:
(B) 29.4 mL
Step-by-step explanation:
1 L = 1000 mL
1:200 solution implies the
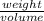 in 200 mL solution.
in 200 mL solution.
200 mL of solution = 1g of Benzalkonium chloride
1000 mL will be
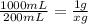
200mL × 1g = 1000 mL × x(g)
x(g) =
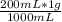
x(g) = 0.2 g
That is to say, 0.2 g of benzalkonium chloride in 1000mL of diluted solution of 1;200 is also the amount in 10mL of the stock solution to be prepared.
∴
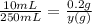
y(g) =
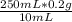
y(g) = 5g of benzalkonium chloride.
Now, at 17%
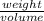 concentrate contains 17g/100ml:
concentrate contains 17g/100ml:
∴ the number of milliliters of a 17% benzalkonium chloride stock solution that is needed to prepare a liter of a 1:200 solution of benzalkonium chloride will be;
=
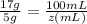
z(mL) =
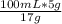
z(mL) = 29.41176 mL
≅ 29.4 mL
Therefore, there are 29.4 mL of a 17% benzalkonium chloride stock solution that is required to prepare a liter of a 1:200 solution of benzalkonium chloride