Solution:
Given:
initial orbit of electron=
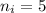
final orbit of electron=
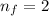
to find wavelength we use Rydberg equation:
1/λ
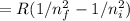
where R is Rydberg constant and it's value is
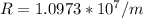
inserting all the values in formula
1/λ
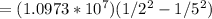
λ
for it's region and color look at given attached pictures