Answer: The molal concentration of KOH (aq) is 0.8
Step-by-step explanation:
Depression in freezing point:
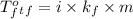
where,
 = freezing point of solution
= freezing point of solution
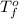 = freezing point of solvent
= freezing point of solvent
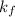 = freezing point constant
= freezing point constant
m = molality
i = Van't Hoff factor = number of ions produced on dissociation
For
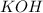 i =2 as
i =2 as
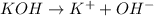
For
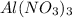 i =4 as
i =4 as
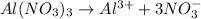
Now put all the given values in the above formula, we get:
For 0.4
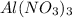
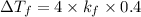
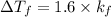
For KOH
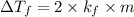
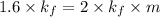
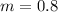
Thus molal concentration of KOH (aq) is 0.8