Answer:
The answer to the question which of the following statements is TRUE is
c. Rate constants are temperature dependent.
Step-by-step explanation:
The rate constant can be derived from the following Arrhenius equation thus
k =
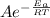
Where A = Arrhenius constant
R = Universal gas constant
T= Absolute temperature
Eₐ = Activation energy of the reaction
The above equation makes it clear that the rate of a reaction is mainly impacted by the temperature of the reaction.
The rate constant therefore is not actually a constant as it is only valid for a specified temperature