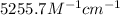The question is incomplete, here is the complete question:
A murexide soution has a concentration of
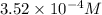 and an absorbance of 1.850 and the path length is 1 cm. What is the molar absorptivity of the solution?
and an absorbance of 1.850 and the path length is 1 cm. What is the molar absorptivity of the solution?
Answer: The molar absorptivity coefficient is
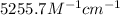
Step-by-step explanation:
To calculate the molar absorptivity coefficient, we use the equation given by Beer-Lambert law, which is:
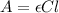
where,
A = absorbance = 1.850
 = molar absorptivity coefficient = ?
= molar absorptivity coefficient = ?
C = concentration of the solution =
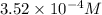
l = path length = 1 cm
Putting values in above equation, we get:
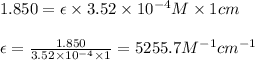
Hence, the molar absorptivity coefficient is