Step-by-step explanation:
As the cross-section of wire is a square so, it means that height of the wire is same length as the width.
Now, we will convert both height and width into centimeters as follows.
h = w
=
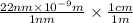
=
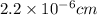
Length of wire will also be converted into centimeters as follows.
l =
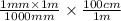
= 0.1 cm
Now, we will calculate the volume of the wire as follows.
V =
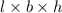
=
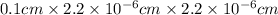
=
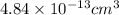
Now, we will convert moles of copper into atoms of copper as follows.
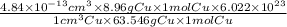
=
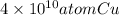
Thus, we can conclude that there will be
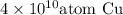 would be in a length of such an interconnect, assuming a square cross section.
would be in a length of such an interconnect, assuming a square cross section.