Answer : The energy required to melt 58.3 g of solid n-butane is, 4.66 kJ
Explanation :
First we have to calculate the moles of n-butane.
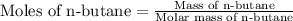
Given:
Molar mass of n-butane = 58.12 g/mole
Mass of n-butane = 58.3 g
Now put all the given values in the above expression, we get:
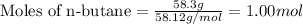
Now we have to calculate the energy required.
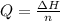
where,
Q = energy required
 = enthalpy of fusion of solid n-butane = 4.66 kJ/mol
= enthalpy of fusion of solid n-butane = 4.66 kJ/mol
n = moles = 1.00 mol
Now put all the given values in the above expression, we get:
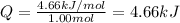
Thus, the energy required to melt 58.3 g of solid n-butane is, 4.66 kJ