Answer:
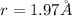
Step-by-step explanation:
First of all, we need to find the mass in grams per atom. We can divide the standard atomic weight by the Avogadro constant.
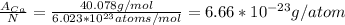 (1)
(1)
We know the calcium crystallizes in a face-centered cubic unit cell, so we can find the mass of the 4 calcium atoms in the face-centered cubic unit cell. We just need to multiply the value calculated above times 4.
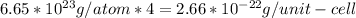 (2)
(2)
We can calculate the volume of this unit cell using the density value.
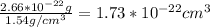
Whit this value, let's find the edge length (L) of this unit cell:
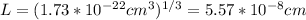
Let's remember that a face-centered unit cell has an atom in the middle of each cube's face, so if we see the diagonal of one face of this cube, we realize that its length is equal to 4 radii of the calcium atom. So, we will have a triangle, where each cathetus is L and the hypotenuse is 4r (r is the radius of the atom). Using the Pythagorean Theorem we will find r.
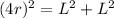
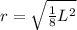
Therefore, the radius of the calcium atom is:
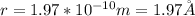
I hope it helps you!