Answer:
The 13.76% of propionic acid is in the dissociated form in the solution
Step-by-step explanation:
Concentration of propionic acid = c = 0.61mM =
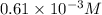
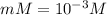
Degree of dissociation = α
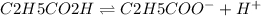
At initial
c 0 0
At equilibrium
c - cα cα cα
The value of dissociation constant of propionic acid =
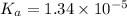
The expression of dissociation constant of propionic acid is given by :
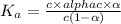
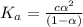
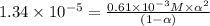
Solving the equation for
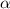 :
:
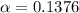
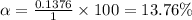
The 13.76% of propionic acid is in the dissociated form in the solution