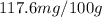Answer: The solubility of nitrogen gas at 5.00 atm is
Step-by-step explanation:
To calculate the molar solubility, we use the equation given by Henry's law, which is:
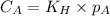
Or,
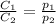
where,
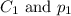 are the initial concentration and partial pressure of nitrogen gas
are the initial concentration and partial pressure of nitrogen gas
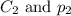 are the final concentration and partial pressure of nitrogen gas
are the final concentration and partial pressure of nitrogen gas
We are given:
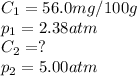
Putting values in above equation, we get:
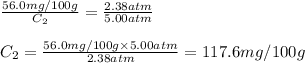
Hence, the solubility of nitrogen gas at 5.00 atm is