Answer:
After 120.0 minutes the the concentration of reactant A will be 0.175 M
Step-by-step explanation:
Rate constant of the reaction = k =
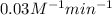
From the units of rate constant is very much obvious that reaction of second order kinetics.
Initial concentration of reactant =
![[A_o]=0.50M](https://img.qammunity.org/2021/formulas/chemistry/college/cs2nw99tpl899aanqgggmribajjmu64fx5.png)
Final concentration of A after time t =
![[A]=0.175 M](https://img.qammunity.org/2021/formulas/chemistry/college/r7jj201135sokodqoy9t6hgmhrtf2t4hxo.png)
t = ?
Integrated rate law for second order kinetics is given by:
![(1)/([A])=kt+(1)/([A_0])](https://img.qammunity.org/2021/formulas/chemistry/college/4jf8ue7p974iw1er3foniw11g8h91jzpn9.png)
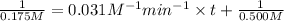
t = 119.8 min ≈ 120.0 min
After 120.0 minutes the the concentration of reactant A will be 0.175 M