Step-by-step explanation:
The given data is as follows.
Enthalpy of vaporization,
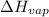 = 38.56 kJ/mol
= 38.56 kJ/mol
Temperature (T) =
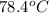
= (78.4 + 273) K
= 351.4 K
Now, we will calculate the change in entropy using the formula as follows.
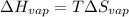
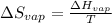
=
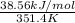
= 0.109 kJ/mol K
Thus, w ecan conclude that the entropy change for vaporization is 0.109 kJ/mol K.