Answer: The amount of heat required is 775.7 kJ
Step-by-step explanation:
The processes involved in the given problem are:
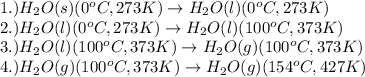
Now, we calculate the amount of heat released or absorbed in all the processes.
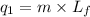
where,
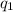 = amount of heat absorbed = ?
= amount of heat absorbed = ?
m = mass of water or ice = 248 g
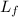 = latent heat of fusion = 334 J/g
= latent heat of fusion = 334 J/g
Putting all the values in above equation, we get:
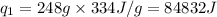
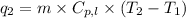
where,
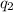 = amount of heat absorbed = ?
= amount of heat absorbed = ?
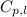 = specific heat of water = 4.184 J/g°C
= specific heat of water = 4.184 J/g°C
m = mass of water = 248 g
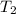 = final temperature =
= final temperature =
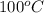
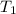 = initial temperature =
= initial temperature =
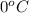
Putting all the values in above equation, we get:
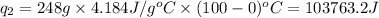
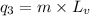
where,
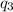 = amount of heat absorbed = ?
= amount of heat absorbed = ?
m = mass of water or ice = 248 g
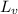 = latent heat of vaporization =
= latent heat of vaporization =
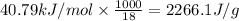 (Conversion factor used: 1 kJ = 1000 J and molar mass of water = 18 g/mol)
(Conversion factor used: 1 kJ = 1000 J and molar mass of water = 18 g/mol)
Putting all the values in above equation, we get:
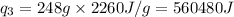
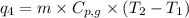
where,
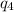 = amount of heat absorbed = ?
= amount of heat absorbed = ?
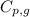 = specific heat of steam = 1.99 J/g°C
= specific heat of steam = 1.99 J/g°C
m = mass of water = 248 g
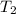 = final temperature =
= final temperature =
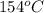
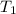 = initial temperature =
= initial temperature =
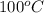
Putting all the values in above equation, we get:
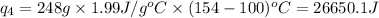
Calculating the total heat absorbed, we get:
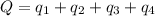
![Q=[84832+103763.2+560480+26650.1]J=775,725.3J=775.7kJ](https://img.qammunity.org/2021/formulas/chemistry/high-school/ibv0uxgrjzh2mscqcg5qd9jwyhrejoss9l.png)
Hence, the amount of heat required is 775.7 kJ