Answer:
59.2 grams
Step-by-step explanation:
We are given that 70.4% of the weight of the total 200 g of the concentration is made up of nitric acid, the remaining information is not required to solve the problem. Since water and nitric acid are the only components of the solution, the total weight of water is given by:
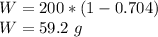
There are 59.2 grams of water in this solution.