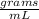Answer:
The density of the metal sample is 2.70
Step-by-step explanation:
Density is a magnitude that allows you to measure the amount of mass in a given volume of a substance. Then, the expression for the calculation of density is the ratio between the mass of a body and the volume it occupies:
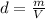
Density, according to the International System of Units, is usually expressed in kilograms per cubic meter (kg / m3) or gram per cubic centimeter (g / cm3). Although it can also be expressed in any other unit of mass by volume.
So, for the calculation of density you know that:
- mass of the sample= 10 grams
- volume of the sample = volume on the cylinder after immersion - volume on the cylinder before immersion= 38.7 mL - 35.0 ml = 3.7 mL
Then:
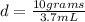
d=2.70
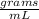
The density of the metal sample is 2.70