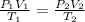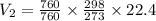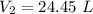Answer:
Step-by-step explanation:
Given
Temperature of gas at First stage
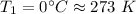
Pressure of gas at First stage
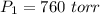
Volume Occupies
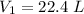
If the Pressure and Temperature at second stage is
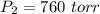
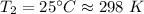
Using ideal gas Equation

where P=Pressure
V=volume
R=Universal Gas constant
T=Temperature
n=no of moles
as n and R is constant therefore
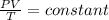
thus