Answer:
Step-by-step explanation:
The table with the pressure and volume values for the particular gas of the question is:
P (pascals) V (liters)
5.0 × 10³ 6.0
1.5 × 10⁴ 3.0
2.0 × 10⁴ 2.0
2.5 × 10⁴ 1.5
Since the pressure of 7.0×10³ pascals is between two of the pressures reported in the table, and there is not any simple equation (inverse proportionaliy or a line) that relates those pressures and volumes, you need to interpolate.
Interpolation is calculating an unknown property assuming that the data in between two known set of properties follow a linear relation.
You need to use the points (6.0, 5.0 × 10³) and (3.0, 1.5 × 10⁴), along with (V, 7.0 × 10³)
Where V is the volume that you need to estimate.
The calculation is:
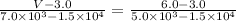
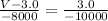
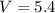
Hence, the best estimate for the value of V at 7.0 × 10³ pascals is 5.5 liters.