Answer:
109.09°C
Step-by-step explanation:
Given that:
the capacity of the cooling car system = 5.6 gal
volume of solute = volume of the water; since a 50/50 blend of engine coolant and water (by volume) is used.
∴
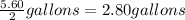
Afterwards, the mass of the solute and the mass of the water can be determined as shown below:
mass of solute =
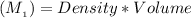
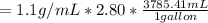
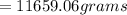
On the other hand; the mass of water =
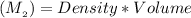
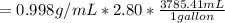
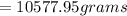
Molarity =
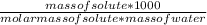
=
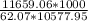
= 17.757 m
≅ 17.76 m
∴ the boiling point of the solution is calculated using the boiling‑point elevation constant for water and the Molarity.
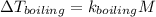
where,
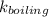 = 0.512 °C/m
= 0.512 °C/m
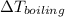 = 100°C + 17.56 × 0.512
= 100°C + 17.56 × 0.512
= 109.09 °C