Answer:
1. 0.27 m
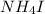 : Highest boiling point
: Highest boiling point
2. 0.11 m
: Lowest boiling point
3. 0.16 m
: Third highest boiling point
4. 0.5 m sucrose : Second highest boiling point
Step-by-step explanation:
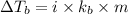
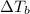 = elevation in boiling point
= elevation in boiling point
i = Van'T Hoff factor
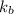 = boiling point constant
= boiling point constant
m = molality
1. For 0.27 m
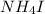
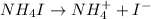
, i= 2 as it is a electrolyte and dissociate to give 2 ions. and concentration of ions will be
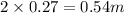
2. For 0.11 m
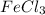
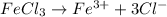
i= 4 as it is a electrolyte and dissociate to give 4 ions. and concentration of ions will be
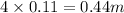
3. For 0.16 m

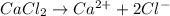
, i= 3 as it is a electrolyte and dissociate to give 3 ions, concentration of ions will be
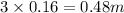
4. For 0.5 m sucrose
, i= 1 as it is a non electrolyte and does not dissociate to give ions, concentration will be 0.5 m
Thus as concentration of solute follows the order :
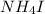 > sucrose >
> sucrose >
 >
>
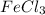 , the boiling point will also follow the same order.
, the boiling point will also follow the same order.