Step-by-step explanation:
First we will convert the given mass from lb to kg as follows.
157 lb =
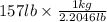
= 71.215 kg
Now, mass of caffeine required for a person of that mass at the LD50 is as follows.
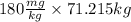
= 12818.7 mg
Convert the % of (w/w) into % (w/v) as follows.
0.65% (w/w) =
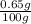
=
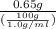
=
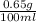
Therefore, calculate the volume which contains the amount of caffeine as follows.
12818.7 mg = 12.8187 g =
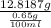
= 1972 ml
Thus, we can conclude that 1972 ml of the drink would be required to reach an LD50 of 180 mg/kg body mass if the person weighed 157 lb.