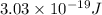Answer : The energy of one photon of hydrogen atom is,
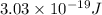
Explanation :
First we have to calculate the wavelength of hydrogen atom.
Using Rydberg's Equation:
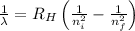
Where,
 = Wavelength of radiation
= Wavelength of radiation
 = Rydberg's Constant = 10973731.6 m⁻¹
= Rydberg's Constant = 10973731.6 m⁻¹
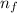 = Higher energy level = 3
= Higher energy level = 3
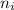 = Lower energy level = 2
= Lower energy level = 2
Putting the values, in above equation, we get:
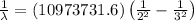
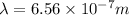
Now we have to calculate the energy.
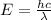
where,
h = Planck's constant =
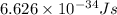
c = speed of light =
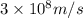
 = wavelength =
= wavelength =
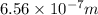
Putting the values, in this formula, we get:
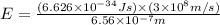
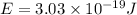
Therefore, the energy of one photon of hydrogen atom is,