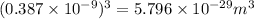Answer:
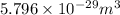
Explanation:
Atomic radius of metal=0.137nm=
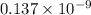 m
m
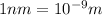
Structure is FCC
We know that
The relation between edge length and radius in FCC structure
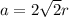
Where a=Edge length=Side
r=Radius
Using the relation
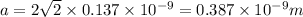
We know that
Volume of cube=
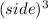
Using the formula
Volume of unit cell=