Answer:
P₂= 141.15 kPa
Step-by-step explanation:
Given that
Volume ,V= 3 L
V= 0.003 m³
Initial temperature ,T₁ = 273 K
Initial pressure ,P₁ = 105 kPa
Final temperature ,T₂ = 367 K
Given that volume of the cylinder is constant .
Lets take final pressure = P₂
We know that for constant volume process
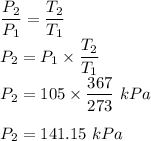
Therefore the final pressure = 141.15 kPa
P₂= 141.15 kPa