Answer:
62.92°
Step-by-step explanation:
given,
initial mass of water, m₁ = 71.8 g
initial temperature, T₁ = 78.8°C
another mass of water, m₂ = 33.6 g
another temperature of water, T₂ = 29° C
Final temperature of mix = ?
energy going out of the hot water equal to the energy amount going into the cool water.
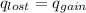
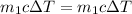
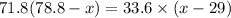
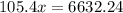
x = 62.92°
Hence, the final temperature of the mix is equal to 62.92°