Answer:
a) The atomic mass of the potassium is 54.23 amu.
b) The melting point of bromine gas is 6.3°C.
Step-by-step explanation:
a) Atomic mass of Na =22.99 amu
Atomic mass of Rb = 85.47 amu
Döbereiner triad = Na , K ,Rb
Taking average of atomic masses of Na and Rb
Atomic mass of the K =
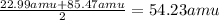
The atomic mass of the potassium is 54.23 amu.
b) Melting point of chlorine gas =-101.0°C
Melting point of iodine gas =113.6°C
Döbereiner triad = Cl, Br , I
Melting point of bromine gas :
=
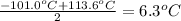
The melting point of chlorine gas is 6.3°C.