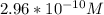The question happens to be in an incorrect order but the correct question can be seen below;
What concentration of
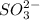 is in equilibrium with
is in equilibrium with
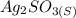 and
and
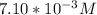
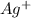 ? (The
? (The
 of
of
Answer:
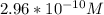
Step-by-step explanation:
The concentration of
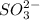 can be determined by using the solubility concept.
can be determined by using the solubility concept.
Given ionic solid is
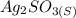 ;
;
The Equilibrium Equation for the ionic compound will be:
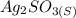 ⇄
⇄
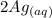 +
+
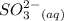
Now, the solubility product (
 ) of the ionic compound will be;
) of the ionic compound will be;

![= [Ag^+]^(2)[SO^(2-)_3]](https://img.qammunity.org/2021/formulas/chemistry/college/d1s4yo1btaj8tvp7bvbo0f1rc35iwkvmy2.png)
Given that;
the concentration
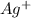 is
is
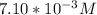 ; &
; &
solubility product of the given ionic solid is
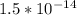
∴

![= [Ag^+]^(2)[SO^(2-)_3]](https://img.qammunity.org/2021/formulas/chemistry/college/d1s4yo1btaj8tvp7bvbo0f1rc35iwkvmy2.png)
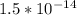
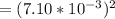
![[SO^(2-)_3]](https://img.qammunity.org/2021/formulas/chemistry/college/65s717qce1lv56iulsvzdnl653s4dh8xky.png)
![[SO^(2-)_3]](https://img.qammunity.org/2021/formulas/chemistry/college/65s717qce1lv56iulsvzdnl653s4dh8xky.png) =
=
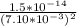
= 2.97560008 × 10⁻¹⁰
≅
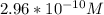
Thus, the concentration of
![[SO^(2-)_3]](https://img.qammunity.org/2021/formulas/chemistry/college/65s717qce1lv56iulsvzdnl653s4dh8xky.png) is
is