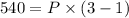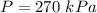Answer:
Step-by-step explanation:
Given
Initial Volume
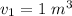
final Volume
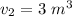
Heat added at constant Pressure
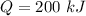
Decrease in Energy of System
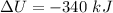
According to First law of thermodynamics
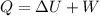
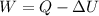
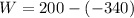
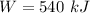
Work done in a constant Pressure Process is given by
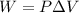
where P is the constant Pressure