Answer : The volume of stock solution needed will be, 2.53 mL
Explanation : Given,
Stock solution = 178 mg/mL
Working solution = 45 mg/mL
Volume of working solution = 10 mL
Formula used :
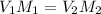
where,
 = concentration of Stock solution
= concentration of Stock solution
 = concentration of working solution
= concentration of working solution
 = volume of stock solution
= volume of stock solution
 = volume of working solution
= volume of working solution
Now put all the given values in the above formula, we get:
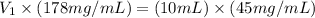
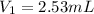
Thus, the volume of stock solution needed will be, 2.53 mL