Answer: The equilibrium concentration of sulfur dioxide, nitrogen dioxide, sulfur trioxide, nitrogen monoxide is 0.196 M, 0.196 M, 0.309 M and 0.309 M respectively.
Step-by-step explanation:
We are given:
Initial concentration of sulfur dioxide = 0.500 M
Initial concentration of nitrogen dioxide = 0.500 M
Initial concentration of sulfur trioxide = 0.00500 M
Initial concentration of nitrogen monoxide = 0.00500 M
The chemical reaction follows:
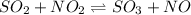
Initial: 0.500 0.500 0.005 0.005
At eqllm: 0.500-x 0.500-x 0.005+x 0.005+x
The expression of equilibrium constant for the above reaction follows:
![K_c=([SO_3][NO])/([SO_2][NO_2])](https://img.qammunity.org/2021/formulas/chemistry/college/euu15xnqjw4x9pq5k7l6y6rzbweklg5k4s.png)
We are given:
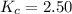
Putting values in above equation, we get:
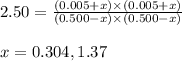
Neglecting the value of x = 1.37, because change cannot be greater than the initial concentration
So, equilibrium concentration of sulfur dioxide =
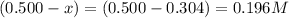
Equilibrium concentration of nitrogen dioxide =
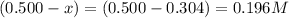
Equilibrium concentration of sulfur trioxide =
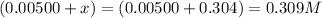
Equilibrium concentration of nitrogen monoxide =
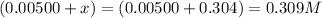
Hence, the equilibrium concentration of sulfur dioxide, nitrogen dioxide, sulfur trioxide, nitrogen monoxide is 0.196 M, 0.196 M, 0.309 M and 0.309 M respectively.