Answer:
A) 0.162 M
Step-by-step explanation:
Calculation of the moles of
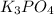 as:-
as:-
Mass = 3.00 g
Molar mass of
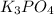 = 212.27 g/mol
= 212.27 g/mol
The formula for the calculation of moles is shown below:
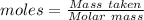
Thus,
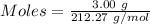
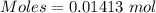
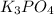 will form ions as:-
will form ions as:-
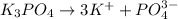
Total number of ions = 3 + 1 = 4
Total moles =
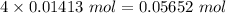
Volume = 350 mL = 0.35 L ( 1 mL = 0.001 L )
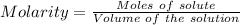
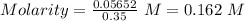
Total concentration of the ions - A) 0.162 M