Answer:
321 mL of 78% chlorine solution must be mixed with 156.16 mL of 23% chlorine solution
Explanation:
Let
x ----> quantity of 78% chlorine solution in milliliters
y ----> quantity of 23% chlorine solution in milliliters
we know that
The quantity x of 78% chlorine solution mixed with a quantity y of 23% chlorine solution produce 477.16 mL of 60% solution

so
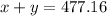 ----> equation A
----> equation A
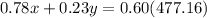
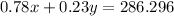 ----> equation B
----> equation B
Solve the system by graphing
Remember that the solution is the intersection point both graphs
using a graphing tool
The solution is the point (321, 156.16)
therefore
321 mL of 78% chlorine solution must be mixed with 156.16 mL of 23% chlorine solution