Answer: 2.068*
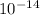 m
m
Explanation: According to work energy-theorem , the workdone in accelerating the electron equals the energy it would give off in terms of light.
workdone= qV
energy = hc/λ
q=magnitude of an electronic charge= 1.602*
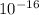
h= planck constant = 6.626*
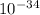
c= speed of light =2.998*
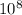
v= potential difference= 6*
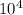
λ= wavelength=unknown
by making λ subject of formulae we have that
λ=
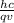
λ = 6.626*
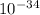 * 2.998*
* 2.998*
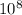 / 1.602*
/ 1.602*
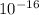 * 6*
* 6*
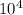
λ =
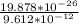
by doing the necessary calculations, we have that
λ = 2.068*
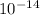 m
m