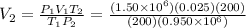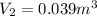Answer:
The final volume is
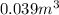
Step-by-step explanation:
Data:
Initial temperature:
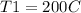
Final temperature:
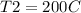
Initial pressure:
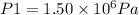
Final pressure:
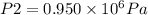
Initial volume:
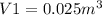
Final volume:
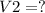
Assuming hydrogen gas as a perfect gas it satisfies the perfect gas equation:
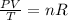 (1)
(1)
With P the pressure, V the volume, T the temperature, R the perfect gas constant and n the number of moles. If no gas escapes the number of moles of the gas remain constant so the right side of equation (1) is a constant, that allows to equate:
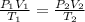
Subscript 2 referring to final state and 1 to initial state.
solving for V2: