Amount of tin produced is 10grams.
Option D.
Step-by-step explanation:
To calculate this problem, we need to know what is molecular weight. Molecular weight of a substance is defined as the weight of a mole of the molecules of that substance.
Here in the reaction, we can see that for every 3 moles of iron sulphide produced, the moles of tin produced is 2.
So for every 0.122 moles of iron sulphide, the number of moles of tin produced is
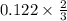 =0.081 moles of tin.
=0.081 moles of tin.
Molecular weight of tin is 121.76grams.
So 0.081mole of tin weighs
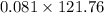 grams =9.91 grams ~10grams.
grams =9.91 grams ~10grams.
So amount of tin produced is 10grams.