The question is incomplete , complete question is :
Microwave radiation has a wavelength on the order of 1.0 cm. Calculate the frequency and the energy of a single photon of this radiation. Calculate the energy of an Avogadro’s number of photons (called an einstein) of this electromagnetic radiation.
Answer:
The energy of an Avogadro’s number of photons of this electromagnetic radiation is 11.97 Joules.
Step-by-step explanation:
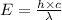
where,
E = energy of photon = ?
h = Planck's constant =
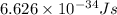
c = speed of light =
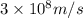
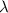 = wavelength =
= wavelength =
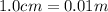
Now put all the given values in the above formula, we get the energy of the photons.
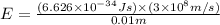
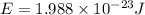
Energy of the 1 mole of photons = E'
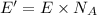
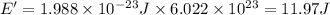
The energy of an Avogadro’s number of photons of this electromagnetic radiation is 11.97 Joules.