Answer:
0.135M/s
Step-by-step explanation:
We are given that
Initial rate of reaction=0.0300M/s
We have to find the initial rate if [A] is halved and [B] is tripled.
Initial rate,
![Rate=k[A][B]^2](https://img.qammunity.org/2021/formulas/physics/college/a70moxaqmqivnguqxnuygxhpv7ca2kclvf.png) =0.0300M/s
=0.0300M/s
![[A]'=(1)/(2)[A]](https://img.qammunity.org/2021/formulas/physics/college/x2vu1hbnn87n031u7bvoicejj6u9hm3zk5.png)
![[B]'=3[B]](https://img.qammunity.org/2021/formulas/physics/college/3bow0qqly3eldawxmecbnc2ac2jgu8ah3u.png)
New, initial rate=
![k[A]'[B]'^2](https://img.qammunity.org/2021/formulas/physics/college/6fxfobtkt1zzh2en22zkud2l2isaei1idz.png)
Substitute the values then we get
New, initial rate,Rate=
![k* (1)/(2)[A]* (3[B])^2](https://img.qammunity.org/2021/formulas/physics/college/5m1vi3fzouiniknd4fi9inx0d8baqzwuxg.png)
New, initial rate
Rate=
![4.5k[A][B]](https://img.qammunity.org/2021/formulas/physics/college/vyxin8zhrgot2myibwcyg1sunlgicune4f.png)
New initial rate=
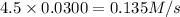
Hence, the initial rate will be 0.135M/s when [A] is halved and [B] is tripled.