Answer:
Empirical formula is
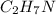 and it is Ethylamine.
and it is Ethylamine.
Step-by-step explanation:
Given that,
A substance consists of 53.5% C, 15.5% H, and 31.19% N by weight. We need to find the empirical formula of a substance.
The atomic masses of each component are as follows :
Carbon, C = 12.011 g/mol
Hydrogen, H = 1.00794 g/mol
Nitrogen, N = 14.01 g/mol
Percentage of carbon,
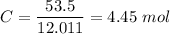
Percentage of hydrogen,
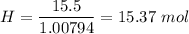
Percentage of nitrogen,
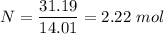
To get the empirical formula of the substance, we divide each element by the lowest number of moles i.e. 2.22 mole
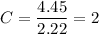
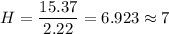
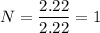
So, there are 2 molecules of carbon, 7 molecules of hydrogen and 1 molecule of nitrogen. Hence, the empirical formula of a substance is
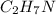 and it is Ethylamine. Hence, this is the required solution.
and it is Ethylamine. Hence, this is the required solution.