Answer:
24555 years
Step-by-step explanation:
Data provided in the question:
Half life of carbon 14 = 5700 years
carbon present in the dead man = 5% of the original amount of carbon 14
Now,
let the initial Amount of carbon be 'A'
Therefore,
after half life
Amount of carbon =
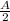
Thus,
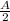 =
=
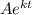
here,
k is the decay constant
t is the time = 5700 years
therefore,
1 =
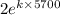
or
0.5 =
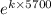
taking natural log both the sides
-0.69314 = 5700k
or
k = - 0.000122
therefore,
for dead man Amount of carbon = 5% of A = 0.05A
thus,
0.05A =
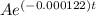
or
0.05 =
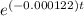
taking natural log both the sides
we get
-2.995 = - 0.000122 × t
or
t = 24555.18 ≈ 24555 years