Step-by-step explanation:
Formula to calculate the atomic packing factor is as follows.
APF =
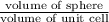
=
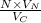
It is given that atomic radius is 0.123 nm and density of metal is 7.45
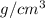 .
.
Hence, number of atoms will be calculated as follows.
No. of atoms =
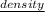 = 4
= 4
APF =
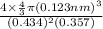
= 1.3905
Thus, we can conclude that atomic packing factor if the unit cell has a tetragonal symmetry is 1.3905.