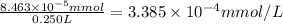Answer:
Concentration of DDT in milli moles per Liters :
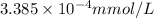 .
.
Step-by-step explanation:
Mass of DDT in sample = 0.030 mg = 0.030 × 0.001 g = 0.00003 g
1 mg = 0.001 g
Molar mass of DDT = 354.5 g/mol
Moles of DDT sample =
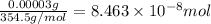
1 mol = 1000 milli mol
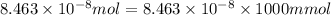
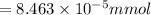
Volume of the sample = 250.0 mL = 0.250 L (1 mL=0.001 L)
Concentration of DDT in milli moles per Liters :