Answer: The pH of the buffer is 5.25
Step-by-step explanation:
Let the volume of buffer solution be V
We know that:
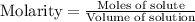
To calculate the pH of acidic buffer, we use the equation given by Henderson Hasselbalch:
![pH=pK_a+\log(\frac{[\text{conjugate base}]}{[acid]})](https://img.qammunity.org/2021/formulas/chemistry/college/rhn1wwm8yavpyps3py0ah7tbd8ay6pteqx.png)
We are given:
 = negative logarithm of acid dissociation constant of weak acid = 4.90
= negative logarithm of acid dissociation constant of weak acid = 4.90
![[\text{conjugate base}]=(2.25)/(V)](https://img.qammunity.org/2021/formulas/chemistry/college/ywxk3vr3d1q693v5ggilx0upvljuceo1vi.png)
![[acid]=(1.00)/(V)](https://img.qammunity.org/2021/formulas/chemistry/college/u4owhfvykx97eyq47m227zbtcs7u19t9ea.png)
pH = ?
Putting values in above equation, we get:
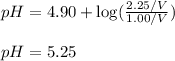
Hence, the pH of the buffer is 5.25