Step-by-step explanation:
According to Lewis, acids are the species which readily accept electrons. Whereas bases are the species which donate electrons to electron deficient or electropositive ions.
Out of the given options, boron of
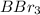 and carbon of
and carbon of
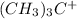 contain sextet of electrons. This means that they have accepted electrons from the combining atoms.
contain sextet of electrons. This means that they have accepted electrons from the combining atoms.
Thus, we can conclude that out of the given options
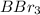 and
and
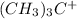 compounds can be labeled as Lewis acids.
compounds can be labeled as Lewis acids.