Answer:
η = 40 %
Step-by-step explanation:
Given that
Qa ,Heat addition= 1000 J
Qr,Heat rejection= 600 J
Work done ,W= 400 J
We know that ,efficiency of a engine given as
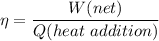
Now by putting the values in the above equation ,then we get
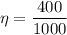
η = 0.4
The efficiency in percentage is given as
η = 0.4 x 100 %
η = 40 %
Therefore the answer will be 40%.