The question is incomplete, here is the complete question:
At 20°C the vapor pressure of benzene
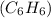 is 75 torr, and that of toluene
is 75 torr, and that of toluene
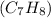 is 22 torr. Assume that benzene and toluene form an ideal solution.
is 22 torr. Assume that benzene and toluene form an ideal solution.
What is the mole fraction of benzene in the solution that has a vapor pressure of 38 torr at 20°C? Express your answer using two significant figures.
Answer: The mole fraction of benzene is 0.302
Step-by-step explanation:
Let the mole fraction of benzene be 'x' and that of toluene is '1-x'
To calculate the total pressure of the mixture of the gases, we use the equation given by Raoult's law, which is:
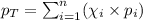
We are given:
Vapor pressure of benzene = 75 torr
Vapor pressure of toluene = 22 torr
Vapor pressure of solution = 38 torr
Putting values in above equation, we get:
![38=[(75* x)+(22* (1-x))]\\\\x=0.30](https://img.qammunity.org/2021/formulas/chemistry/college/hgvgqo665cmtt3fuq8p1wlh1qjwinbstfj.png)
Hence, the mole fraction of benzene is 0.30