Step-by-step explanation:
The given data is as follows.
Density of gasoline = 0.77 g/ml
Volume of gasoline = 35 L = 35000 ml (as 1 L = 1000 ml)
As we know that density of a substance is equal to its mass divided by its volume.
Mathematically, Density =

Hence, calculate the mass of given gasoline as follows.
Density =

0.77 g/ml =
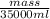
mass = 26950 g
Also, it is given that 1 g of gasoline on combustion produces 45.0 kJ of energy.
Therefore, energy produced by 26950 g of combustion of gasoline will be as follows.
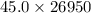
= 1212750 kJ
Thus, we can conclude that the amount of energy produced by burning 35 L of gasoline is 1212750 kJ.