The question is incomplete, here is the complete question:
What is the concentration, in parts per million, of a solution prepared by dissolving 0.00040 mol HCl in 2.2 L
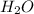 ? Assume that the volume of the solution does not change when the HCl is added. Assume the density of the solution is the same as that for pure water (1.00 g/mL)
? Assume that the volume of the solution does not change when the HCl is added. Assume the density of the solution is the same as that for pure water (1.00 g/mL)
Answer: The concentration of HCl in the solution is 6.64 ppm
Step-by-step explanation:
- To calculate the number of moles, we use the equation:
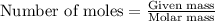
We are given:
Moles of HCl = 0.00040 moles
Molar mass of HCl = 36.5 g/mol
Putting values in above equation, we get:
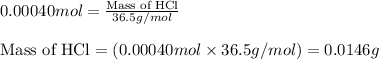
- To calculate the mass of water, we use the equation:
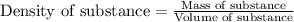
Density of water = 1.00 g/mL
Volume of water = 2.2 L = 2200 mL (Conversion factor: 1 L = 1000 mL)
Putting values in above equation, we get:
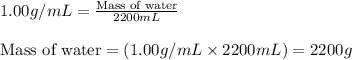
ppm is the amount of solute (in milligrams) present in kilogram of a solvent. It is also known as parts-per million.
To calculate the ppm of HCl in the solution, we use the equation:
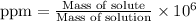
Both the masses are in grams.
We are given:
Mass of HCl = 0.0146 g
Mass of solution = 2200 g
Putting values in above equation, we get:
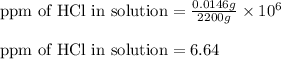
Hence, the concentration of HCl in the solution is 6.64 ppm